News
NEWS
Regrettably, we would like to inform everyone that as of 1st March 2020, our workshop is cancelled due to the outbreak of Coronavirus. For all questions or enquiries, please contact Dr Fabien Delerue or Mrs Amie Law.
GEM is delighted to announce that Macquarie University will be hosting the first iGonad/Easi-CRISPR workhsop in Oceania on 16-18 March 2020!
Prof Gurumurthy Channabasavaiah (University of Nebraska - USA) and Prof Masato Ohtsuka (Tokai University - Japan), inventors of these groundbreaking advancements in genome editing and assisted reproduction will come to Sydney with their teams (Prof Masahiro Sato, Dr Hiromi Miura and Mr Rolen Quadros) to teach their techniques to the attendees. This 3-day workshop will consist of lectures and hands-on practice sessions.
Places are limited so don't miss out!!
To register, and for more information, please contact Dr Fabien Delerue or Mrs Amie Law.
SPONSORS

We thank our valuable sponsors for their support
Some reagents have been generously donated by Integrated DNA Technologies, New England Biolabs and Abacus dx.
PROGRAM
The workshop will include lectures and demonstrations of CRISPR mouse genome engineering experiments using the advanced techniques Easi-CRISPR (Efficient additions with ssDNA inserts CRISPR) and iGONAD (Genome editing via Oviductal Nucleic Acids Delivery). The participants will have ample opportunities to closely interact with the instructors and perform hands-on experiments such as designing CRISPR genome engineering projects, synthesising CRISPR and donors reagents for microinjection and electroporation (iGONAD) experiments, and either watch or perform microinjection and/or iGONAD procedures.
Venue:
- Lectures, computer practice, coffee breaks and lunch: MUSE, 18 Wally's Walk
- Lab sessions: 2 Technology Place, Level 1
- iGonad: Central Animal Facility (CAF)
Monday 16 March 2020
9:00-9:15am Welcome - Fabien
9:15-10:00am “Introduction and overview of workshop agenda” - Guru
10:00-10:15am Coffee Break
10:15-11:15am Lab session: set up PCR reactions and cast agarose gels
11:15- 12:00pm Student short introductions/research focus/experience; preferably present 2-3 slides each, or self-introduction (without slides).
12:00-1:00pm Lunch break
1:00-2:30pm Lab session: gel analysis and purification of PCR products, setting up RNA synthesis
2:30-3:30pm “Basics and advances in CRISPR mouse genome engineering methods - Guru
3:30-3:45pm Coffee break
3:45-4:00pm DNAse treatment
4:00-5:00pm “i-GONAD; a novel genome engineering method” - Masato
5:00-7:00pm Purification of RNA, setting up RT-PCR and cast gels for ssDNA
Tuesday 17 March 2020
8:30-9:00am ssDNA electrophoresis
9:00-9:30am “CUY21EDIT II, constant current electroporator for i-GONAD” sponsored by Bex - Yasuhiro
9:30-10:00am “Genome editing across species by direct electroporation of embryos” sponsored by Nepagene - Fabien
10:00-10:15am Coffee break
10:15-11:30pm Gel isolation, quantification of ssDNA, start an analytic gel to test the final ssDNA prep
11:30-12:00pm “Easy and efficient gene editing using chemically synthesized guide RNA” - Bernice Tan, sponsored by Merck Australia
12:00-12:15pm Imaging agarose gel (of ssDNA electrophoresis)
12:15-1:15pm Lunch break
1:15-1:30pm “Many Ways, One Goal: Moving towards efficient methods in creating genetically engineered models in rodents using CRISPR associated method" - Prasanna
1:30-2:30pm “Preparing Easi-CRISPR microinjection mix” - Rolen
2:30-3:00pm Introduction of i-GONAD settings
3:00-3:45pm i-GONAD demonstration by instructors (Masato, Masahiro, Hiromi)
3:45-4:00pm Coffee break
4:00-6:00pm i-GONAD practice (students)
Wednesday 18 March 2020
8:00-9:30am Embryo isolation and embryo thawing (Nicolle & Jess)
9:30-10:00am Harvesting of zygotes from the i-GONAD treated females and observation of fluorescence
10:00-10:15am Coffee break
10:15-11:15am “Design of conditional and knock-in alleles: relevant examples” - Guru
11:15-11:30am Designing guide RNAs - Rolen
11:30-12:30pm Lunch break
12:30-:3:30pm Microinjection demonstration and practice (Nicolle & Rolen).
3:30-3:45pm Coffee break
3:45-6:00pm Group discussion on molecular biology and iGONAD results & design of student’s Easi-CRISPR projects
6:00 pm Closing remarks (Fabien)
PRACTICAL INFORMATION FOR PARTICIPANTS
Location:
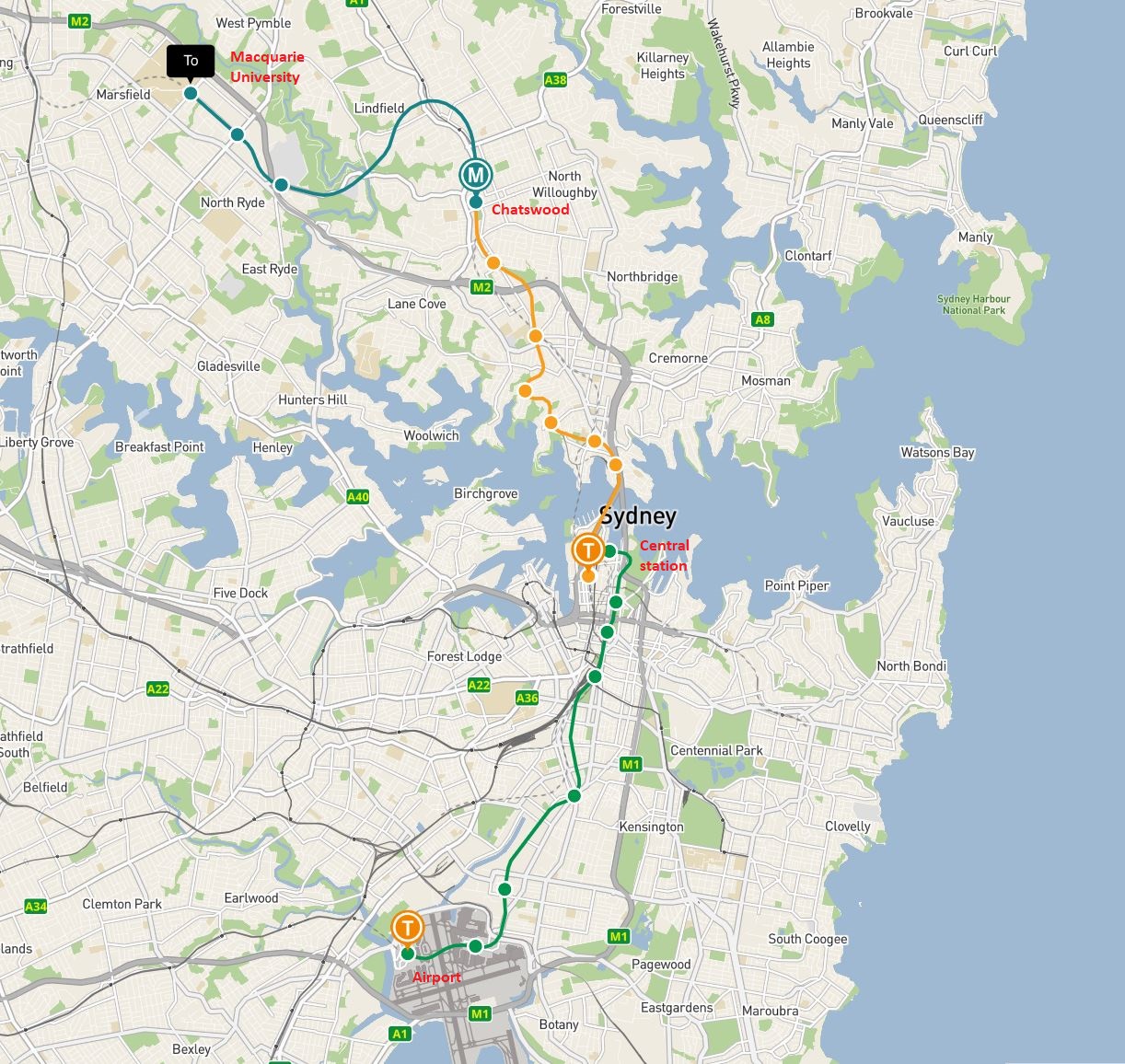
Macquarie University campus is located in Ryde, around 15km North of Sydney CBD and is easily accessible by train or taxi. The trip should approximately take 45min to 1h.
Train: from Sydney Airport, take the train/Metro to Macquarie University Station via Central Station and Chatswood. To organize your transfer, we recommend you visit the TransportNSW website. Trips are paid using Opal cards that you can buy at the Airport.
Taxi: You can easily take a taxi at the airport 24h/7. Uber also operates at the airport. Should you go straight to the Campus, the exact destination is 15 Research Park Drive, Macquarie University NSW 2109. We are located off Talavera road, between the Travelodge (see Accommodation below) and Macquarie Hospital. A map of Macquarie University campus can be found here.
Accommodation:
Accommodation is not included with the registrations, and each participant will need to book their own. Should you like to book a hotel in the CBD, there is a plethora of offers and prices in Sydney. However, there is a 45min/1h ride by train/tram to reach Macquarie University. Should you want to stay closer, we recommend the Travelodge in Macquarie Park, which is next door (literally) to our facilities, and will be our meeting point on Day 1.
Registrations:
Please contact Dr Fabien Delerue to register. Payments will be by card only, and discount applies for ISTT members.
Important considerations before attending the workshop:
International attendees may need to apply a Visa to enter Australia. Usually, an Electronic Travel Authorization (ETA) is sufficient, yet we highly recommend that you check your personal requirements.
During the workshop, fully enclosed shoes MUST be worn at all times. This is a OH&S requirement and can NOT be neglected. Similarly, a 48h exclusion period applies before entering our animal facility. If you have questions or concerns, please contact Dr Fabien Delerue.
Recommended lectures:
The workshop is designed for all, regardless of whether you are new to Easi-CRISPR/iGonad, or whether you want to set up or improve these techniques in your lab. Regardless of your level of knowledge of the technology, we highly recommend that you get familiar with the two recent publications describing the techniques that attendees will implement during the workshop:
Easi-CRISPR for creating knock-in and conditional knockout mouse models using long ssDNA donors. Miura et al. Nat Protoc. 2018 Jan;13(1):195-215. doi: 10.1038/nprot.2017.153.
Creation of CRISPR-based germline-genome-engineered mice without ex vivo handling of zygotes by i-GONAD. Gurumurthy et al. Nat Protoc. 2019 Aug;14(8):2452-2482. doi: 10.1038/s41596-019-0187-x.
Should you be interested in reading more about the development, outcomes and achievements using Easi-CRISPR and/or iGonad in mice and rats, below is a selection of related publications:
Easi-CRISPR:
https://www.ncbi.nlm.nih.gov/pubmed/28511701
https://www.ncbi.nlm.nih.gov/pubmed/29925374
iGonad:
https://www.ncbi.nlm.nih.gov/pubmed/26724720
https://www.ncbi.nlm.nih.gov/pubmed/30104681
https://www.ncbi.nlm.nih.gov/pubmed/29482575