Multiplex Bead Assays
Multiplex assays of biological fluids
Multiplexed immune-assays (MIA)
Multiplexed immuno assays (MIA) are conducted using the Bio-Plex/Luminex suspension array system as a plate reader and epMotion robotic workstation for liquid delivery for precision and reproducibility of the MIA assays. MIA is predominately performed for measuring concentrations of cytokines, chemokines, growth factors, phosphoproteins, and many other human disease specific assays such as cancer biomarker panels, diabetes, metabolic, hormonal, inflammation, cell signalling, kidney toxicity, acute phase, isotyping, etc. from body fluids, cell culture supernatants and tissue samples. In addition to human assays there are several animal assays are available such as canine kidney toxicity, mouse and rat cytokines, etc. MIA is performed at APAF using commercially available assay kits only. There are numerous analytes from different companies who makes kits for MIA partnering with Luminex. There is a Kit finder site where you can search ever-growing commercially available assay kits: http://www.luminexcorp.com/kitfinder
APAF’s automated liquid delivery system facilitates high reproducibility of the assays. Our intra-plate technical reproducibility (% CV) is 2.6 and inter-plate is 7.6 which were observed from 27-plex 19 assay plates and assays were conducted over two weeks’ period.
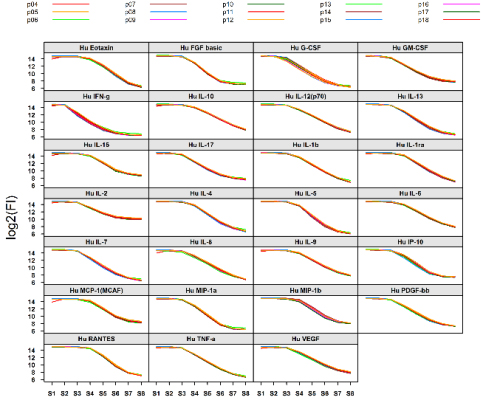
Reproductivity of MIA data at APAF. Serially diluted 27 cytokine standards from 19 assay plates were overlaid showed an excellent reproducibility of each cytokine. Assays were conducted over two week’s period using the epMotion robotic liquid handling workstation and a magnetic plate washer.
APAF’s Biostatisticians, experts on MIA data analysis, are available for your data analysis needs and interpretation of results. They can also help in planning an experiment to ensure that sufficient sample size and statistical power are available to answer specific research questions. Our Biostatisticians use the latest statistical, modelling procedures and global analysis strategies, which ensure the lowest possible false positive and negative rates. These procedures and algorithms are different compared to conventional statistical and stratification methods, and often reveal deeper analytical results with higher statistical power. Consult our recent publications on MIA data analysis.
If you wish to see published articles on research done with these kits a good place to start is the Luminex Blog where they put up published papers for each month using bead technologies.
https://www.luminexcorp.com/blog
Contact us for estimated cost prior to sending samples to APAF.
Key benefits of multiplex assay
- Multiplexing up to 100 bioassays per well (per sample); internal controls allowable in each well.
- Using up to 50 µL of sample obtains up to 100 results.(sample size may be less and is dependent on the kit insert)
- Quantitative results over a 3 to 4 log dynamic range, with strong concordance to ELISA and Mass Spectrometry.
- All the liquid handling operations for cytokine assays performed on a robotic motion system for precise and reproducible liquid delivery in an automatic fashion.
Co-efficient of Variation (CV) of technical replicates is low in the bead based immunoassays. Assays in duplicate or triplicate would be sufficient. However, increasing biological replicates would give better statistical significance.
Sample Preparation guidelines
For serum/plasma or other body fluids and cell culture samples:
- protease inhibitor cocktails into the samples if samples cannot be frozen soon after collection.
- Centrifuge samples at 20,000 g for 5 min; discard pellets (bottom) and lipid (top) from the samples for plasma/serum samples. Use the middle part for MIA. For cell culture samples, use the supernatant.
- Filter samples through 0.22 µm spin filters. This is very important for reproducibility and assay bead counts. APAF can spin filter samples for customers with an additional cost.
- Ship samples (100 -200 µ/sample) to APAF on dry ice. It requires 75 µl per replicate if the Robotic Liquid Handling Workstation is used for liquid delivery (50 µl per replicate is required if the assay performed by hand). If more than one plate to analyse from the same samples, increase the volume of samples accordingly.
- For cell culture supernatants, approximately 5 ml of cell culture medium itself must send to APAF together with the samples. This is required for preparation of standards
How do I send sample from overseas to APAF?
If you are sending any biological samples to APAF from countries outside of Australia go to the Quarantine Guidelines in our website or contact us in regards to permits needed for Australian quarantine regulations and the recommended couriers to use.
Pathogenicity
Biological samples coming into APAF should be accompanied by documentation of potential pathogenicity or pathogen free status otherwise APAF will presume all samples from human and animal origin are potential pathogens and will be treated accordingly.
Multiplex Analysis Using Bio-Plex/Luminex System Service Request Form
Once you have followed all the guidelines you can download the Multiplex Analysis Using Bio-Plex/Luminex System Service Request Form (PDF 358KB) from the website.Fill this form and send to APAF together with the samples.
