Post Translational Modification - Phosphorylation
Measuring Protein Phosphorylation – Can APAF help you?
Protein phosphorylation is a signalling mechanism that regulates most aspects of cellular life. The enzymes responsible for protein phosphorylation, the protein kinases constitute one of the largest family of enzymes encoded by the human genome. Regulation of protein function by phosphorylation is a fundamental mechanism used by eukaryotic organisms. Phosphorylations are a rapid chemical way to either activate or deactivate the protein substrates. Dysregulation of protein phosphorylation is commonly associated with disease. For example, in the case of cancer, mutations in regulatory kinases (e.g. BRAF) can result in hyper-phosphorylation of substrates, effectively jamming on signalling pathways that lead to cell proliferation.
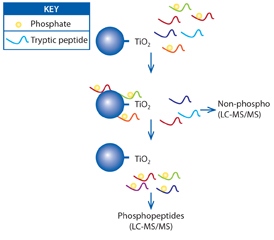
Phosphoprotein analysis presents numerous analytical challenges. Typically, phosphopeptides are present in low abundance compared with non-phosphorylated counterparts. Phosphopeptides are often highly charged and hydrophilic which can impact on analysis strategies. Determining the site of phosphorylation on peptides by mass spectrometry is often complicated. Nowadays, robust methods have been established to conduct global analysis of protein phosphorylation – phosphoproteomics. At APAF we have established methods to enrich and analyse phosphopeptides with the use of high resolution mass spectrometry. For Phospho-serine/threonine peptides we use titanium dioxide based enrichment. For phosphotyrosine containing peptides immunoaffinity capture is used. APAF has found that TiO2 enrichments provide a solution to detect thousands of phosphopeptides from complex cell lysates. Orbitrap or Q-ToF mass spectrometers are used to provide high confidence in peptide detection.
In recent work using CPA cells derived from Barrett’s oesophagus treated with PLK inhibitors supplied by a pharmaceutical company, APAF was able to identify many hundreds of singly, doubly and triply phosphorylated tryptic phosphopeptides.